Chicken intestinal organoids: a novel method to measure the mode of action of feed additives
There is a rapidly growing interest in how the avian intestine is affected by dietary components and feed additives.
The paucity of physiologically relevant models has limited research in this field of poultry gut health and led to an over-reliance on the use of live birds for experiments.
The development of complex 3D intestinal organoids or "mini-guts" has created ample opportunities for poultry research in this field. A major advantage of the floating chicken intestinal organoids is the combination of a complex cell system with an easily accessible apical-out orientation grown in a simple culture medium without an extracellular matrix.
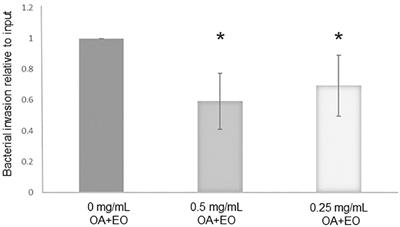
The objective was to investigate the impact of a commercial proprietary blend of organic acids and essential oils (OA+EO) on the innate immune responses and kinome of chicken intestinal organoids in a Salmonella challenge model. To mimic the in vivo prolonged exposure of the intestine to the product, the intestinal organoids were treated for 2 days with 0.5 or 0.25 mg/mL OA+EO and either uninfected or infected with Salmonella and bacterial load in the organoids was quantified at 3 hours post infection.
The bacteria were also treated with OA+EO for 1 day prior to challenge of the organoids to mimic intestinal exposure.
The treatment of the organoids with OA+EO resulted in a significant decrease in the bacterial load compared to untreated infected organoids.
The expression of 88 innate immune genes was investigated using a high throughput qPCR array, measuring the expression of 88 innate immune genes. Salmonella invasion of the untreated intestinal organoids resulted in a significant increase in the expression of inflammatory cytokine and chemokines as well as genes involved in intracellular signalling. In contrast, when the organoids were treated with OA+EO and challenged with Salmonella, the inflammatory responses were significantly downregulated.
The kinome array data suggested decreased phosphorylation elicited by the OA+EO with Salmonella in agreement with the gene expression data sets. This study demonstrates that the in vitro chicken intestinal organoids are a new tool to measure the effect of the feed additives in a bacterial challenge model by measuring innate immune and protein kinases responses.
Read the full article at the original website
References: