Pfizer Whistleblower Sinks Vaccine Trial Integrity
According to a whistleblower who worked on Pfizer’s Phase 3 COVID jab trial, data were falsified, patients were unblinded, the company hired poorly trained people to administer the injections, and follow-up on reported side effects lagged way behind

Yet again, mainstream media have completely ignored what should have been front-page news. According to a whistleblower who worked on Pfizer’s Phase 3 COVID jab trial in the fall of 2020, data were falsified, patients were unblinded, the company hired poorly trained people to administer the injections, and follow-up on reported side effects lagged way behind.
What makes the media’s silence all the more remarkable is that this revelation was published in The British Medical Journal. Paul Thacker, investigative journalist for The BMJ, writes in his November 2, 2021, report:1
1“Revelations of poor practices at a contract research company helping to carry out Pfizer’s pivotal covid-19 vaccine trial raise questions about data integrity and regulatory oversight ...
“Revelations of poor practices at a contract research company helping to carry out Pfizer’s pivotal covid-19 vaccine trial raise questions about data integrity and regulatory oversight ...
[F]or researchers who were testing Pfizer’s vaccine at several sites in Texas during that autumn, speed may have come at the cost of data integrity and patient safety ... Staff who conducted quality control checks were overwhelmed by the volume of problems they were finding.”
[F]or researchers who were testing Pfizer’s vaccine at several sites in Texas during that autumn, speed may have come at the cost of data integrity and patient safety ... Staff who conducted quality control checks were overwhelmed by the volume of problems they were finding.”
As noted by Bill Bruckner for transparimed.org:2
2“Editors’ widespread failure to pick up on the story is deeply problematic. First and foremost, it lets the U.S. Food and Drug Administration off the hook for what appear to be severe lapses in regulatory oversight over this trial ... Where are the media outlets questioning the FDA about its oversight processes? Where are the politicians calling for an enquiry? ...
“Editors’ widespread failure to pick up on the story is deeply problematic. First and foremost, it lets the U.S. Food and Drug Administration off the hook for what appear to be severe lapses in regulatory oversight over this trial ... Where are the media outlets questioning the FDA about its oversight processes? Where are the politicians calling for an enquiry? ...
Second, it lets Pfizer off the hook for apparently failing to adequately oversee the operations of its subcontractor ... Where are the media outlets questioning Pfizer about its oversight and quality assurance processes? ...
Second, it lets Pfizer off the hook for apparently failing to adequately oversee the operations of its subcontractor ... Where are the media outlets questioning Pfizer about its oversight and quality assurance processes? ...
Third, it undermines confidence in democratic institutions and public health bodies because it gives citizens ... the impression that mainstream media are deliberately ignoring a big story in order to avoid fueling vaccine hesitancy.”
Third, it undermines confidence in democratic institutions and public health bodies because it gives citizens ... the impression that mainstream media are deliberately ignoring a big story in order to avoid fueling vaccine hesitancy.”
So far, this story has been largely confined to the alternative news media. You’ll find a selection of video reports covering the whistleblower’s testimony in the sections below.
The whistleblower in question is Brook Jackson, a former regional director of Ventavia Research Group, a research organization charged with testing Pfizer’s COVID jab at several sites in Texas.
Jackson repeatedly “informed her superiors of poor laboratory management, patient safety concerns and data integrity issues,” Thacker writes, and when her concerns were ignored, she finally called the U.S. Food and Drug Administration and filed a complaint via email.
Jackson was fired later that day after just two weeks on the job. According to her separation letter, management decided she was “not a good fit” for the company after all. She has provided The BMJ with “dozens of internal company documents, photos, audio recordings and emails” proving her concerns were valid. According to Jackson, this was the first time she’d ever been fired in her 20-year career as a clinical research coordinator. Thacker explains:3
3“Jackson was a trained clinical trial auditor who previously held a director of operations position and came to Ventavia with more than 15 years’ experience in clinical research coordination and management.
“Jackson was a trained clinical trial auditor who previously held a director of operations position and came to Ventavia with more than 15 years’ experience in clinical research coordination and management.
Exasperated that Ventavia was not dealing with the problems, Jackson documented several matters late one night, taking photos on her mobile phone. One photo, provided to The BMJ, showed needles discarded in a plastic biohazard bag instead of a sharps container box.
Exasperated that Ventavia was not dealing with the problems, Jackson documented several matters late one night, taking photos on her mobile phone. One photo, provided to The BMJ, showed needles discarded in a plastic biohazard bag instead of a sharps container box.
Another showed vaccine packaging materials with trial participants’ identification numbers written on them left out in the open, potentially unblinding participants ... Jackson told The BMJ that drug assignment confirmation printouts were being left in participants’ charts, accessible to blinded personnel ...
Another showed vaccine packaging materials with trial participants’ identification numbers written on them left out in the open, potentially unblinding participants ... Jackson told The BMJ that drug assignment confirmation printouts were being left in participants’ charts, accessible to blinded personnel ...
In a recording of a meeting in late September 2020 between Jackson and two directors a Ventavia executive can be heard explaining that the company wasn’t able to quantify the types and number of errors they were finding when examining the trial paperwork for quality control. ‘In my mind, it’s something new every day,’ a Ventavia executive says. ‘We know that it’s significant.’
In a recording of a meeting in late September 2020 between Jackson and two directors a Ventavia executive can be heard explaining that the company wasn’t able to quantify the types and number of errors they were finding when examining the trial paperwork for quality control. ‘In my mind, it’s something new every day,’ a Ventavia executive says. ‘We know that it’s significant.’
Ventavia was not keeping up with data entry queries, shows an email sent by ICON, the contract research organization with which Pfizer partnered on the trial. ICON reminded Ventavia in a September 2020 email: ‘The expectation for this study is that all queries are addressed within 24hrs.’
Ventavia was not keeping up with data entry queries, shows an email sent by ICON, the contract research organization with which Pfizer partnered on the trial. ICON reminded Ventavia in a September 2020 email: ‘The expectation for this study is that all queries are addressed within 24hrs.’
ICON then highlighted over 100 outstanding queries older than three days in yellow. Examples included two individuals for which ‘Subject has reported with Severe symptoms/reactions … Per protocol, subjects experiencing Grade 3 local reactions should be contacted. Please confirm if an UNPLANNED CONTACT was made and update the corresponding form as appropriate.’
ICON then highlighted over 100 outstanding queries older than three days in yellow. Examples included two individuals for which ‘Subject has reported with Severe symptoms/reactions … Per protocol, subjects experiencing Grade 3 local reactions should be contacted. Please confirm if an UNPLANNED CONTACT was made and update the corresponding form as appropriate.’
According to the trial protocol a telephone contact should have occurred ‘to ascertain further details and determine whether a site visit is clinically indicated.’ Documents show that problems had been going on for weeks.
According to the trial protocol a telephone contact should have occurred ‘to ascertain further details and determine whether a site visit is clinically indicated.’ Documents show that problems had been going on for weeks.
In a list of ‘action items’ circulated among Ventavia leaders in early August 2020, shortly after the trial began and before Jackson’s hiring, a Ventavia executive identified three site staff members with whom to ‘Go over e-diary issue/falsifying data, etc.’ One of them was ‘verbally counseled for changing data and not noting late entry,’ a note indicates.”
In a list of ‘action items’ circulated among Ventavia leaders in early August 2020, shortly after the trial began and before Jackson’s hiring, a Ventavia executive identified three site staff members with whom to ‘Go over e-diary issue/falsifying data, etc.’ One of them was ‘verbally counseled for changing data and not noting late entry,’ a note indicates.”
In her complaint to the FDA, Jackson listed a dozen incidents of concern, including the following:
- Participants were not monitored by clinical staff after receiving the shot
- Patients who experienced adverse effects were not promptly evaluated
- Protocol deviations were not being reported
- The Pfizer injection vials were stored at improper temperatures
- Laboratory specimens were mislabeled
Not a single one of the problems Jackson raised in her complaint to the FDA were noted or addressed in Pfizer’s briefing document submitted to the FDA’s advisory committee meeting December 20, 2020, when its emergency use authorization application was reviewed.
The FDA went ahead, granting the Pfizer jab emergency use authorization the very next day, despite being in receipt of Jackson’s complaint, which ought to have put the brakes on the FDA’s authorization. At bare minimum, they should have investigated the matter before proceeding.
What’s more, the FDA’s summary of its inspections of the Pfizer trial, published in August 2021, revealed the agency only inspected nine of the 153 trial sites, and Ventavia was not one of them. The complaint also appears to have been ignored when the FDA granted full approval to Comirnaty, Pfizer/BioNTech’s COVID shot that is not yet available.
Pfizer is also in on the cover-up. Shortly after Jackson’s firing, Pfizer was notified of the problems she’d raised. Despite that, Pfizer has since then contracted Ventavia to conduct no less than four additional trials — one for COVID shots in children and young adults, one for the COVID jab in pregnant women, a booster shot trial, and an RSV vaccine trial.
So, clearly, Pfizer is not opposed to contractors falsifying data or otherwise undermining the integrity of the trials. That alone is a fiery indictment against Pfizer.
They can feign ignorance and proclaim to adhere to “the highest scientific, ethical and clinical standards”4 all they want. Those are just words which, unless backed by consistent action, are completely meaningless. Behind the scenes, they’re clearly well-aware that their trials are resting on fraudulent foundations.
4Jackson wasn’t the only employee to get sacked from Ventavia after raising concerns about the integrity of the Pfizer trial. Thacker writes:5
5“In recent months Jackson has reconnected with several former Ventavia employees who all left or were fired from the company. One of them was one of the officials who had taken part in the late September meeting. In a text message sent in June the former official apologized, saying that ‘everything that you complained about was spot on.’
“In recent months Jackson has reconnected with several former Ventavia employees who all left or were fired from the company. One of them was one of the officials who had taken part in the late September meeting. In a text message sent in June the former official apologized, saying that ‘everything that you complained about was spot on.’
Two former Ventavia employees spoke to The BMJ anonymously for fear of reprisal and loss of job prospects in the tightly knit research community. Both confirmed broad aspects of Jackson’s complaint.
Two former Ventavia employees spoke to The BMJ anonymously for fear of reprisal and loss of job prospects in the tightly knit research community. Both confirmed broad aspects of Jackson’s complaint.
One said that she had worked on over four dozen clinical trials in her career, including many large trials, but had never experienced such a ‘helter skelter’ work environment as with Ventavia on Pfizer’s trial. ‘I’ve never had to do what they were asking me to do, ever,’ she told The BMJ. ‘It just seemed like something a little different from normal — the things that were allowed and expected’ ...
One said that she had worked on over four dozen clinical trials in her career, including many large trials, but had never experienced such a ‘helter skelter’ work environment as with Ventavia on Pfizer’s trial. ‘I’ve never had to do what they were asking me to do, ever,’ she told The BMJ. ‘It just seemed like something a little different from normal — the things that were allowed and expected’ ...
After Jackson left the company problems persisted at Ventavia, this employee said. In several cases Ventavia lacked enough employees to swab all trial participants who reported COVID-like symptoms, to test for infection. Laboratory confirmed symptomatic COVID-19 was the trial’s primary endpoint, the employee noted.
After Jackson left the company problems persisted at Ventavia, this employee said. In several cases Ventavia lacked enough employees to swab all trial participants who reported COVID-like symptoms, to test for infection. Laboratory confirmed symptomatic COVID-19 was the trial’s primary endpoint, the employee noted.
(An FDA review memorandum released in August this year states that across the full trial swabs were not taken from 477 people with suspected cases of symptomatic COVID-19.) ‘I don’t think it was good clean data,’ the employee said of the data Ventavia generated for the Pfizer trial. ‘It’s a crazy mess.’”
(An FDA review memorandum released in August this year states that across the full trial swabs were not taken from 477 people with suspected cases of symptomatic COVID-19.) ‘I don’t think it was good clean data,’ the employee said of the data Ventavia generated for the Pfizer trial. ‘It’s a crazy mess.’”
Such statements clearly fly in the face of statements made by world leaders, health authorities and the mainstream media. Most, like federal health minister for Australia, Greg Hunt, have claimed the COVID shots have undergone “rigorous, independent testing” to ensure they’re “safe, effective and manufactured to a high standard.”6
6Nothing we know so far supports such a conclusion. The testing has been far from rigorous and has not been independently verified.
Vaccine Adverse Events Reporting System (VAERS) data show they’re shockingly far from safe; real-world data show effectiveness wanes within a handful of months while leaving you more susceptible to SARS-CoV-2 variants and other infections; and manufacturing standards have also been shown lacking, as a variety of foreign contaminants have been found in the vials.7
7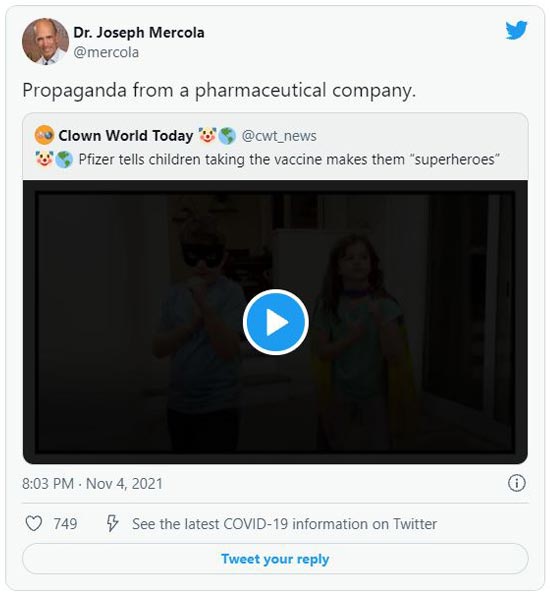
One of the reasons why English- and German-speaking legacy media have completely ignored this whistleblower testimony is probably because Pfizer has such a dominating influence over them. Thacker told blogger Maryanne Demasi, Ph.D.,8 “Pfizer has such a huge PR machine, they have basically captured the media, they’ve hypnotized the media.”
8Pfizer’s PR department is also hard at work trying to hypnotize the public. The TV ad above is perhaps one of the most offensive. In it, Pfizer brainwashes young children into thinking that getting the COVID shot will make them superheroes. Never mind the fact that getting the shot could kill or permanently injure them.
The video at the top of this article is a short extract from a November 2, 2021, meeting organized by Sen. Ron Johnson, during which associate editor of The BMJ, Peter Doshi, Ph.D., reviewed some of the many concerns experts have about the integrity of the COVID jab data.
He points out that Pfizer’s raw trial data will not be made available until May 2025. So far, Pfizer has refused to release any of its raw data to independent investigators and, without that, there’s no possible way to confirm that what Pfizer is claiming is actually true and correct.
Without data, it’s not science. ~ Peter Doshi, Ph.D., associate editor of The British Medical Journal
In other words, we’re expected to simply take the word of a company that has earned a top spot on the list of white collar criminals; a company that in 2009 was fined a record-breaking $2.3 billion in fines for fraudulent marketing and health care fraud.9 Press releases are not science. They’re marketing. Without the raw data, we have no science upon which to base our decisions about the COVID shot.
9As noted by Dr. Robert Kaplan from Stanford’s School of Medicine Clinical Excellence Research Centre, who also spoke at the meeting:
“The evidence we have comes from highly curated, industry-controlled press releases and journal publications. We are making big decisions based on limited, highly selected evidence. A compromised scientific process will lead to poor decisions, and it may set a bad precedent.”
“The evidence we have comes from highly curated, industry-controlled press releases and journal publications. We are making big decisions based on limited, highly selected evidence. A compromised scientific process will lead to poor decisions, and it may set a bad precedent.”
Doshi stresses how utterly unscientific a process we’re now following. He also points out that doctors have an ethical duty to not recommend a treatment for which they have no data. Quoting from a 2020 article he co-wrote:10
10“Data transparency is not a ‘nice to have.’ Claims made without access to the data — whether appearing in peer reviewed publications or in preprints without peer review — are not scientific claims.
“Data transparency is not a ‘nice to have.’ Claims made without access to the data — whether appearing in peer reviewed publications or in preprints without peer review — are not scientific claims.
Products can be marketed without access to the data, but doctors and professional societies should publicly state that, without complete data transparency, they will refuse to endorse COVID-19 products as being based on science.”
Products can be marketed without access to the data, but doctors and professional societies should publicly state that, without complete data transparency, they will refuse to endorse COVID-19 products as being based on science.”
“The point I am trying to make is very simple,” Doshi said. “The data from COVID vaccines are not available and won’t be available for years. Yet, we are not just ‘asking’ but ‘mandating’ millions of people to take these vaccines ... Without data, it’s not science.”
We’ve known the FDA is a captured agency for at least a decade. None of the issues we’re now seeing are exactly new. We’re now getting a close-up view of just how dangerous the incestuous relationship between the FDA and Big Pharma really is.
Americans are dying from COVID jab injuries at unprecedented record rates, and the FDA is completely ignoring it. Instead, it continues to push for more jabs, more injuries and more deaths. It’s complicit in causing avoidable deaths rather than protecting public health. That’s the price we’re now paying for not cleaning up the agency and sealing the revolving door between regulators and industry earlier.
In “Designed to Fail: Why Regulatory Agencies Don’t Work,”11 published in May 2012 — nearly a decade ago — William Sanjour discussed the failures of regulatory reform. He points out that the reason reforms don’t work is because they keep reforming in the wrong direction:
11“... as a result of the recent catastrophic failures of regulatory agencies, politicians and pundits are talking about the same old ‘Regulatory Reform’ again. ‘Fill the regulatory agencies with honest people who won’t cave in to special interests.’ ‘Give them more money, more authority and more people.’
“... as a result of the recent catastrophic failures of regulatory agencies, politicians and pundits are talking about the same old ‘Regulatory Reform’ again. ‘Fill the regulatory agencies with honest people who won’t cave in to special interests.’ ‘Give them more money, more authority and more people.’
But my experience has shown that by concentrating all legislative, executive and judiciary authority in one regulatory agency just makes it easier for it to be corrupted by the industries it regulates.
But my experience has shown that by concentrating all legislative, executive and judiciary authority in one regulatory agency just makes it easier for it to be corrupted by the industries it regulates.
I worked for the U.S. Environmental Protection agency for 30 years and lived through many cycles of ‘Regulatory Reform,’ doing the same ‘reforms’ over and over again and expecting different results.
I worked for the U.S. Environmental Protection agency for 30 years and lived through many cycles of ‘Regulatory Reform,’ doing the same ‘reforms’ over and over again and expecting different results.
I’ve learned that the way to achieve true regulatory reform is to give regulatory agencies less money, less authority, fewer people but more intelligent regulations. The theme of this article is that by dispersing regulatory authority, rather than concentrating it, we would make corruption more difficult and facilitate more sensible regulation.”
I’ve learned that the way to achieve true regulatory reform is to give regulatory agencies less money, less authority, fewer people but more intelligent regulations. The theme of this article is that by dispersing regulatory authority, rather than concentrating it, we would make corruption more difficult and facilitate more sensible regulation.”
Sanjour points out that regulators being captured by the parties they’re supposed to regulate is far more dangerous than having no regulatory agencies at all, because “capture gives industry the power of government.” Can there be any doubt that the FDA, as an agency captured by Big Pharma in general and Pfizer in particular, now wields power over the U.S. government?
“From my own experience with the U.S. EPA, even if an inspector finds a violation, this only triggers a lengthy complex process with many levels of warning, review, appeal, negotiation, and adjudication before any action is taken (or, more often, avoided),” Sanjour writes.12
“From my own experience with the U.S. EPA, even if an inspector finds a violation, this only triggers a lengthy complex process with many levels of warning, review, appeal, negotiation, and adjudication before any action is taken (or, more often, avoided),” Sanjour writes.12
12“See the labyrinthine flow chart13 for an example of an agency enforcement procedure. It resembles a game of ‘chutes and ladders.’ Compare this with what happens when you park under a ‘No Parking’ sign. A policeman writes a ticket, and you can either pay the fine or tell it to the judge.
“See the labyrinthine flow chart13 for an example of an agency enforcement procedure. It resembles a game of ‘chutes and ladders.’ Compare this with what happens when you park under a ‘No Parking’ sign. A policeman writes a ticket, and you can either pay the fine or tell it to the judge.
13If the EPA wrote the rules for parking violations, the officer would first have to determine if there were sufficient legal parking available at a reasonable cost and at a reasonable distance, and would then have to stand by the car and wait until the owner showed up so that he could negotiate a settlement agreement.”
If the EPA wrote the rules for parking violations, the officer would first have to determine if there were sufficient legal parking available at a reasonable cost and at a reasonable distance, and would then have to stand by the car and wait until the owner showed up so that he could negotiate a settlement agreement.”
Even more disturbing, Sanjour reveals that, when he was writing regulations for the EPA, he was “told on more than one occasion to make sure I put in enough loopholes. The purpose of the complexity is to hide the loopholes.” Sanjour went on to explain:
“Regulatory agencies are created by Congress in order to control some powerful forces in society (usually corporations), which benefit society but which are also prone to abuse their power. The purpose of a regulatory agency is to allow the flow of benefits while straining out the abuse.
“Regulatory agencies are created by Congress in order to control some powerful forces in society (usually corporations), which benefit society but which are also prone to abuse their power. The purpose of a regulatory agency is to allow the flow of benefits while straining out the abuse.
In order to do this, Congress gives administrators of regulatory agencies broad discretionary power to write regulations for industries for which they are responsible. The flaw in the system is that the administrator is appointed by the president ... Thus any discretionary authority given to a regulatory agency administrator is, in fact, given to the president of the United States to be used as the president sees fit ...
In order to do this, Congress gives administrators of regulatory agencies broad discretionary power to write regulations for industries for which they are responsible. The flaw in the system is that the administrator is appointed by the president ... Thus any discretionary authority given to a regulatory agency administrator is, in fact, given to the president of the United States to be used as the president sees fit ...
[R]egulatory agencies, by their very nature, can do little that doesn’t adversely affect business, especially big and influential business, and this disturbs a president’s repose.
[R]egulatory agencies, by their very nature, can do little that doesn’t adversely affect business, especially big and influential business, and this disturbs a president’s repose.
The EPA, for instance, cannot write regulations governing the petroleum industry without the oil companies going to the White House screaming ‘energy crisis!’ ... When the FDA wants to thoroughly evaluate a new drug, the pharmaceutical company lets loose a public relations barrage about how the bureaucratic delays are costing lives.
The EPA, for instance, cannot write regulations governing the petroleum industry without the oil companies going to the White House screaming ‘energy crisis!’ ... When the FDA wants to thoroughly evaluate a new drug, the pharmaceutical company lets loose a public relations barrage about how the bureaucratic delays are costing lives.
Regulatory agency employees soon learn that drafting and implementing rules for big corporations means making enemies of powerful and influential people. They learn to be ‘team players,’ an ethic that permeates the entire agency without ever being transmitted through written or even oral instructions.
Regulatory agency employees soon learn that drafting and implementing rules for big corporations means making enemies of powerful and influential people. They learn to be ‘team players,’ an ethic that permeates the entire agency without ever being transmitted through written or even oral instructions.
People who like to get things done, who need to see concrete results for their efforts, don’t last long. They don’t necessarily get fired, but they don’t advance either; their responsibilities are transferred to others, and they often leave the agency in disgust. The people who get ahead are those clever ones with a talent for procrastination, obfuscation, and coming up with superficially plausible reasons for accomplishing nothing.”
People who like to get things done, who need to see concrete results for their efforts, don’t last long. They don’t necessarily get fired, but they don’t advance either; their responsibilities are transferred to others, and they often leave the agency in disgust. The people who get ahead are those clever ones with a talent for procrastination, obfuscation, and coming up with superficially plausible reasons for accomplishing nothing.”
The question staring us in the face now is, how do we fix these regulatory agencies so that they can operate for the benefit of the public rather than private for-profit interests?
“The reason salaried government regulators can be corrupted is that writing and enforcing effective regulations is not their No. 1 priority,” Sanjour noted. “Their main objective is keeping their job and advancing their careers.” Industries, meanwhile, believe that pressuring corrupt officials is the only way to protect their business. The answer, Sanjour suggests, is:
“… to remove discretionary judgment from the hands of the regulatory bureaucracy and place it in hands less susceptible to industry influence. The first thing I would suggest is to make use of people or institutions who have a vested interest in effective regulation as strong or stronger than the regulated community.”
“… to remove discretionary judgment from the hands of the regulatory bureaucracy and place it in hands less susceptible to industry influence. The first thing I would suggest is to make use of people or institutions who have a vested interest in effective regulation as strong or stronger than the regulated community.”
Sanjour cites research showing that, by far, whistleblowers — who risk their jobs by speaking out — are the No. 1 fraud detection group, responsible for 19% of frauds being brought to light. The U.S. Securities and Exchange Commission, meanwhile, which exists to uncover corporate fraud, was responsible for just 7%.
So, one way we could improve the system is by issuing monetary rewards to corporate whistleblowers. “Monetary rewards for whistleblowers pay benefits far in excess of the cost when compared with hired regulatory bureaucrats,” Sanjour notes. Insurance companies can also play an important role, as they are far less likely to overlook safety shortcuts that can result in disaster. An example given by Sanjour is the BP oil spill:
“BP has admitted, between 2005 and 2010, to breaking U.S. environmental and safety laws and committing outright fraud and paid $373 million in fines. Between June 2007 and February 2010, BP refineries in Texas and Ohio accounted for 97% of the ‘egregious, willful’ violations handed out by the U.S. Occupational Safety and Health Administration. Yet none of this resulted in any oversight of the Deepwater Horizon oil rig that blew up ...
“BP has admitted, between 2005 and 2010, to breaking U.S. environmental and safety laws and committing outright fraud and paid $373 million in fines. Between June 2007 and February 2010, BP refineries in Texas and Ohio accounted for 97% of the ‘egregious, willful’ violations handed out by the U.S. Occupational Safety and Health Administration. Yet none of this resulted in any oversight of the Deepwater Horizon oil rig that blew up ...
If BP had been required to carry a $10 billion insurance policy for an oil spill, I’m sure the insurance company would not have allowed the penny-pinching short cuts that the paid regulators allowed. If the laws are written intelligently, insurance companies can be a significant instrument for regulation.”
If BP had been required to carry a $10 billion insurance policy for an oil spill, I’m sure the insurance company would not have allowed the penny-pinching short cuts that the paid regulators allowed. If the laws are written intelligently, insurance companies can be a significant instrument for regulation.”
A third group that makes for a far better fraud detection system than federal regulators is the public. Organizations such as Citizens for Health and Environmental Justice teaches citizens how to get involved in the enforcement of regulations, and even more can be done in that regard.
For example, the EPA could sponsor civilian testing and equip citizens living in polluted areas with resources to conduct their own testing and report back if toxic exposures are found. Sanjour continues:14
14“A second reform I would suggest for removing discretionary authority from the regulatory bureaucracy is to make the rules as simple as possible and to place all appellate functions and consent agreements into the hands of the law courts, just as in our traffic cop example.
“A second reform I would suggest for removing discretionary authority from the regulatory bureaucracy is to make the rules as simple as possible and to place all appellate functions and consent agreements into the hands of the law courts, just as in our traffic cop example.
This could be judicial courts or administrative law courts. Anything to take the discretionary authority away from the people who write and enforce the rules and provide one more barrier to industry influence.”
This could be judicial courts or administrative law courts. Anything to take the discretionary authority away from the people who write and enforce the rules and provide one more barrier to industry influence.”
To do any or all of that, we first need to reorganize our regulatory agencies in accordance with the U.S. Constitution. As explained by Sanjour, the U.S. has three branches of government: the legislative, executive and judicial branches. However, when regulatory agencies were formed, we diverted from this structure.
Regulations are a type of laws, and as such they should come from the legislative branch. But regulatory agencies are part of the executive branch. Judicial functions have also been usurped by regulatory agencies, and hence the executive branch.
“Thus, despite the wishes of the Founding Fathers, the executive branch now includes a great many regulatory agencies whose functions span all three branches of government. A large part of the corruption and inefficiency noted above flows from this fact,” Sanjour notes.
“Thus, despite the wishes of the Founding Fathers, the executive branch now includes a great many regulatory agencies whose functions span all three branches of government. A large part of the corruption and inefficiency noted above flows from this fact,” Sanjour notes.
While making changes such as those proposed by Sanjour sounds simple enough, the political pushback would be enormous, and would have to be broken through, somehow. Legally, however, it would be a reasonably simple affair.
All Congress would need to do is amend the law such that the agency administrator is stripped of its authority to write rules and implement the law. That authority would then be transferred to another agency, the administrator of which would be appointed by Congress, not the president.
“Note that these are all paper changes. They do not require any relocation, new buildings, new hires, etc. The functions all currently exist. They are merely rearranged,” Sanjour says.
At present, we can no longer overlook the FDA’s corruption. It’s costing too many lives. They have completely abandoned any semblance of working for the public good. How we get rid of them and fix the problem will become an increasingly pressing question as we move forward.
Read the full article at the original website.
References:
- 1, 3, 5 The BMJ 2021; 375:n2635
- 2 Transparimed.org November 4, 2021
- 4 Pfizer Research Integrity & Transparency
- 6 Twitter Greg Hunt February 15, 2021
- 7 CNBC August 30, 2021
- 8 Maryannedemasi.com November 3, 2021
- 9 ABC Australia September 4, 2009
- 10 The BMJ 2020; 370; m3260
- 11, 12, 14 Independent Science News May 1, 2012
- 13 Independent Science News RCRA C&E Process Flow Chart